|
| |||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Note to the Reader This is a revised edition of a paper published in Neuroscience in 1984. Revised HTML edition <http://www.nervenet.org/papers/Binoc84.html> copyright © 1998 by Robert W. Williams
Print Friendly Neuroscience Vol. 12, No. 4, PP. 1139—1146, 1984
Introduction
During fetal development of the cat’s visual system there is a marked
overproliferation of optic nerve axons. Binocular interactions before
birth contributes to the severity of fiber loss since removal of an eye
during gestation attenuates axon loss in the remaining optic nerve
(Williams et al., 1983; Rakic and Riley, 1983). The purpose of the present
study was to determine whether this reduced loss of optic nerve fibers is
due to a failure of retraction by supernumerary axon branches or to a
reduction in ganglion cell death. To resolve this issue, we compared the
number of ganglion cells and optic nerve fibers in adult cats which had
one eye removed at known gestational ages. Retinal ganglion cells were
backfilled with horseradish peroxidase and counts were made from retinal
wholemounts. The axon complement was assessed with an electron microscopic
assay. In the retinas of a normal cat we estimated 151,000 and 152,000
ganglion cells. The optic nerves of two other normal cats contained
approximately 158,000 and 159,000 axons. In comparison, an animal
enucleated on embryonic day 42 had 180,000 ganglion cells and 178,000
optic nerve fibers, while in an animal enucleated on embryonic day 51 the
corresponding estimates were 182,000 and 190,000. The close agreement
between cell and fiber counts indicates that axonal bifurcation does not
contribute appreciably to the axon surplus in the optic nerve of
prenatally enucleated cats. Retinal projections are distributed more widely early in development
than at maturity (Rakic, 1976, 1977; Cavalcante and Rocha-Miranda, 1978;
Frost and Schneider, 1979; Land and Lund, 1979; Linden et al., 1981;
Sengelaub and Finlay, 1982; Sanderson et al., 1982; Williams and Chalupa,
1982, 1983; Shatz, 1983; Chalupa and Williams, 1984). The events
underlying the restriction of retinal connections to discrete regions
within the visual centers of the brain are poorly understood. However, it
is known that this process is dependent upon binocular interaction since
removal of one eye in fetal monkeys (Rakic, 1981) and cats (Williams and
Chalupa, 1982, 1983; Williams, 1983), as well as in neonatal rodents (Land
and Lund, 1979), results in the maintenance of widespread projections from
the remaining eye.
Experimental ProceduresSurgical technique. The in utero surgical technique and the method used to determine gestational age have been described in previous papers (Williams and Chalupa, 1982, 1983, Williams et al., 1983). An incision was made through the uterus to expose the head of the fetus, the eyelids were parted, and an eye was removed with small curved hemostats. The eyes weighed 90 mg at embryonic day 42 (E42) and 230 mg at E51. Incisions were closed with absorbable suture material, and the litters were allowed to come to term. Parturition was on E63 for the fetus enucleated on E42, and on E64 for that enucleated on E51. Injection protocol. At 10- and 12-months of age, the two experimental animals and a normal adult were prepared for physiological recordings under barbiturate anesthesia. The skull overlying the dorsal lateral geniculate nuclei and the superior colliculi was removed. The animals were paralyzed by infusion with gallamine triethiodide in lactated Ringer’s. The pupils were dilated with homatropine hydrobromide, and the corneas fitted with clear contact lenses. Retinal landmarks were projected onto a tangent screen located 57 cm in front of the animal. A series of penetrations were made with a tungsten microelectrode through the dorsal lateral geniculate nucleus and superior colliculus in order to map these structures and accurately delimit their margins. Subsequently, four injections of horseradish peroxidase (60–80% Sigma Type VI in 2% dimethylsulfoxide) were made into each lateral geniculate nucleus and superior colliculus using a 10 µl syringe fitted with a 27-gauge needle. Each injection of 1.0 µl was delivered continuously over a 15 min period. Histological procedures. Following a survival period which
ranged from 12 to 24 h, animals were anesthetized deeply, and perfused
transcardially with cold saline followed by 2.5% purified glutaraldehyde
and 1.25% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The eye,
optic nerve, optic tracts and brain were removed. Brains were sectioned at
50 µm, and reacted using either the tetramethyl benzidine or the
phenylenediamine-pyrocatechol method (Hanker et al., 1977).
Analysis. Prints of each retina were made at x10 by inserting
the wholemounts into the negative carrier of an enlarger. The retinal
areas were measured using a computerized planimeter (Zeiss Videoplan).
Each retina was scanned at x400 using a planapochromatic oil objective.
Ganglion cells could be identified unequivocally by the dark brown
granules of chromogen that outlined the somata and dendrites. Labeled
neurons were counted within the margins of a net graticule covering an
area of 0.058 mm2. Cells intersecting the upper
and right edges of the graticule were also included in the count. A sample
was taken from each square millimeter of the retina, except around the
area centralis, where samples were taken at 0.25 mm2intervals.
The numbers of labeled cells per sample field were transferred to x10
drawings of the wholemount. Sampling loci with similar count values were
connected to form a set of concentric isodensity lines. These contours
were smoothed by obtaining supplemental samples.
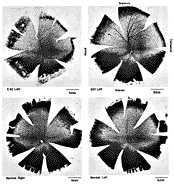
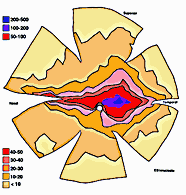
ResultsIn experimental and control animals, peroxidase reaction product filled the entire dorsal lateral geniculate nucleus the superficial portion of the pretectal complex, and the full extent of the superior colliculus on both sides of the brain. In every animal, labeled ganglion cells were distributed across the entire retina, and typical examples of perikaryal label from central and peripheral portions of the retina are depicted in Fig. 1(A) and (B). Low-power micrographs of the left and right retinas from a normal cat and those of the two experimental animals are shown in Fig. 2. Examples of the ganglion cell count distribution and isodensity profile for one of these wholemounts are illustrated in Fig. 3. Prominent features of ganglion cell distribution are readily apparent, including: (i) a dense region of labeled neurons a few millimeters above and temporal to the optic disc which comprises the area centralis; (ii) the horizontal streak, an elongated aggregate of cells extending along the temporal and nasal axis and (iii) a pronounced radial gradient of population density.
The two retinas obtained from the normal cat contained 151,000 and
152,000 ganglion cells. In comparison, we have previously estimated that
the optic nerves of two normal mature animals contained 158,000 and
159,000 fibers (Williams et al., 1983). TABLE 1: Effects of Prenatal Unilateral Enucleation upon Ganglion Cell and Optic Axon Number [updated edition]
*Optic nerve estimates from Williams et al., 1983. All animals
were mature
Are the surplus ganglion cells in the prenatally enucleated animals confined to a particular retinal region? To answer this question we performed a regional analysis of the ganglion cell population for each retina. Three sets of comparisons were made: area centralis vs the periphery, temporal vs nasal retinal fields, and the region of the decussation line (a 2-mm-wide strip centered over the border between temporal and nasal sides of the retina) vs the remaining portion of the retina. These results, summarized in Table 2, revealed that the population increment was not limited to a particular region of the retina in either experimental animal. In the cat enucleated at the earlier prenatal age (E42) a greater excess of ganglion cells appears to be concentrated in the central region; however, there is considerable variability in the area centralis counts in the normal retinas and, most likely, this reflects the difficulty of obtaining an accurate count in this region due to the high density of labeled cells.
TABLE 2: Assessement of Regional Effects on Cell Density following Prenatal Enucleation
The central retian is defined as the region in which the
ganglion cell density exceeds 50 cells per sample area (0.058 mm2).
DiscussionWe have demonstrated that the increase in the cat’s optic nerve fiber
population that results from prenatal unilateral enucleation is matched
closely by the number of ganglion cells in the remaining eye. In one case
(E42 enucleate) the estimates of optic fibers and ganglion cells diverge
by less than l%, while in the other (E5l enucleate) this difference
amounts to about 4%. This indicates that axonal bifurcation within the
retina or the optic nerve does not contribute significantly to the
over-abundance of optic nerve fibers in unilaterally enucleated cats.
Since the fetal retina of the cat contains many more ganglion cells than
the mature retina (Lia et al., 1983; Stone et al., 1982), we conclude that
prenatal binocular interactions influence ganglion cell survival in the
cat’s retina. Furthermore, the degree to which binocular interactions
regulate the severity of ganglion cell loss does not appear to depend
critically on the gestational age at which one eye is removed. At E42 the
fiber population within the cat’s optic nerve is near its peak or more
than 500,000, while by E51 there are less than 300,000 axons (Williams et
al.,
1983b, [see 1986]). Removal of an eye at these two gestational ages
resulted in essentially the same number of ganglion cells within the
remaining retina. Concluding remarksWhile the results of the present study indicate clearly that prenatal binocular interaction regulates the size of the ganglion cell population, the underlying basis for this interaction remains largely speculative. One obvious possibility is that during early development projections from each eve compete for the control of individual target cells. In the cat a well-defined retinal decussation pattern is present as early as E44: therefore, binocular competition would involve axonal terminals emanating from ganglion cells in the nasal contralateral retina and the temporal ipsilateral retina. The reorganization that has been described in the visual system following early eye removal, including the results of the present study could be interpreted as being due to interruption of an inter-ocular competitive process. In line with the binocular competition hypothesis is the recent finding that some lateral geniculate lateral geniculate neurons in the prenatal cat can be activated by stimulation of both optic nerves. However. anatomical evidence for binocular innervation of individual neurons during fetal development is yet to be provided. At present, therefore, it is equally plausible that removal of one eye makes available additional postsynaptic territory that can be occupied by some of the waiting or later arriving axons derived from ganglion cells of the intact eye. Such an interpretation of binocular interaction also accounts for known consequences of early unilateral monocular enucleation.
AcknowledgementsWe are grateful to Deborah van der List for calculations of cell distribution and the preparation of illustrations. This study was supported by Grant EY03991 from the National Eye Institute to L.M.C., a Jastro Shields Research Award to R.W.W., and Grant G979/49 from the Medical Research Council, London to Z.H.
ReferencesCavalcante L. A. and Rocha-Miranda C. E. (1978) Postnatal development of retinogeniculate, retinopretectal and retinotectal projections in the oppossum. Brain Res. 146, 231–248. Chalupa L. M. and Williams R. W. (1984) Prenatal development and reorganization in the visual system of the cat. In Development of the Visual Pathways in Mammals (eds. Stone J., Dreher B., and Rapaport D.) pp. 89–102, Alan R. Liss. New York. Chalupa L. M. and Williams R. W. (1984) Organization of thc cat’s lateral geniculate nucleus following interruption of prenatal binocular competition. Human Neurobiology.3, 103–107. Dreher B., Potts R. A and Bennett M. R. (1983) Evidence that the early postnatal reduction in number of rat retinal ganglion cells is due to a wave of ganglion cell death. Neurosci. Lett. 36, 255–260. Frost D.O., So K. F. and Schneider G .E. (1979) Postnatal development of retinal projections in the Syrian hamster: a study using autoradiographic and anterograde degeneration techniques. Neuroscience 4, 1649–1677 Hanker J. S., Yates P. E., Metz C. B. and Rustione A. (1977) A new specific sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem. 1. 9, 789–792. Hsaio K. and Schneider G. E. (1980) The effect of early unilateral eye enucleation on bilaterally projecting retinal ganglion cells in hamster. Neurosci. Abstr. 6: 684 Hughes A. (1975) A quantitative analysis of the cat retina ganglion cell topography. J. Comp. Neurol, 163. 107–128. Hume R. I. and Purves D. (1981) Geometry of neonatal neurones and the regulation of synapse elimination. Nature 293, 469–471. Illing R.-B. and Wässle H. (1981) The retinal projection to the thalamus in the cat: a quantitative investigation and a comparison with the retinotectal pathway. J. Comp. Neurol, 202, 265–285. Innocenti G. M. (1981) Growth and reshaping of axons in the establishment of visual callosal connections. Science 212, 824–826. Ivy G. O., Akers R. M. and Killackey H. P. (1979) Differential distribution of callosal projection neurons in the neonatal and adult rat. Brain Res. 173, 532–537. Jackson H. and Parks T. N. (1982) Functional synpase elimination in the developing avian cochlear nucleus with simultaneous reduction in cochlear nerve axon branching. J. Neurosci. 2, 1735–1743. Jeffery G. and Perry V. H. (1981 Evidence for ganglion cell death during development of the ipsilateral retinal projection in the rat. Dev. Brain Res. 2, 176–180 Land P. W. and Lund R. D. (1979) Development of the rat’s uncrossed retinotectal pathway and its relation to plasticity. Science 205, 698–700. Lia B., Williams R. W. and Chalupa L. M. (1983) Early development of retinal specialization: the distribution and decussation patterns of ganglion cells in the prenatal cat demonstrated by retrograde peroxidase labeling. Neurosci. Abstr. 9, 702. [Lia B, Williams RW, Chalupa LM (1986) Does axonal branching contribute to the overproduction of optic nerve fibers during early development of the cat’s visual system. Dev. Brain Res. 25, 296–301.] Linden D. C., Guillery R. W. and Cucchiaro J. (1981) The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development J. Comp. Neurol. 220, 189–211. McLoon S. C. (1982) Alterations in precision of the crossed retinotectal projection during chick development. Science 215, 1418–1420. Mesulam M. M. (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J. Histochem. Cytochem. 26, 106–117. Murray M. (1982) A quantitative study of regenerative sprouting by optic axons in goldfish. J. Comp. Neurol. 209, 352–362. Ng A. Y. K. and Stone J. (1982) The optic nerve of the cat: appearance and loss of axons during normal development. Dev. Brain Res. 5, 263–271. Perry V. H. (1982) The ganglion cell layer of the mammalian retina. In Progress in Retinal Research (eds Osborne N. and Chader G.). Vol. 1, pp. 53–80. Pergamon Press, Oxford. Perry V. H. and Linden R. (1982) Evidence for dendritic competition in the developing retina. Nature 297, 683–685. Perry V. H., Henderson Z. and Linden R. (1983) Postnatal changes in retinal ganglion cell and optic axon populations in pigmented rat. J. Comp. Neurol. 219, 356-368. Rager G. and Rager U. (1976) Generation and degeneration of retinal ganglion cells in the chicken. Exp. Brain Res. 25, 551–553. Rakic P. (1976) Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261, 467–471. Rakic P. (1977) Prenatal development of the visual system in the rhesus monkey. Phil. Trans. R. Soc. B 278, 245–260. Rakic P. (1981) Development of visual centers in the primate brain depends on binocular competition before birth. Science 214, 928–931. Rakic P. and Riley K. P. (1983) Overproduction and elimination of retinal axons in the fetal rhesus monkey. Science 219 1441–1444. Rakic P. and Riley K. P. (1983) Regulation of axon number in primate optic nerve by prenatal binocular competition. Nature 305, 135–137. Sanderson K. J., Dixon P. G. and Pearson L. J. (1982) Postnatal development of retinal projections in brushtailed possum Tricchosurus vulpecula. Dev. Brain Res. 5, 161–180. Sengelaub D. R. and Finlay B. L. (1982) Removal of one eye reduces normally occurring cell death in the remaining eye. Science 213, 573–574. Shatz C. J. (1983) The prenatal development of the cat’s retinogeniculate pathway. J. Neurosci. 3, 482–499. Shatz C. J. and Kirkwood P. A. (1984) Prenatal development of functional connections in the cat’s retinogeniculate pathway. J. Neurosci. In press. Sohal G. S. and Weidman T. A. (1978) Development of the trochlear nerve: loss of axons during normal ontogeny. Brain Res. 142, 455–465. Stone J. (1965) A quantitative analysis of the distribution of ganglion cells in the cat’s retina. J. Comp. Neurol. 124, 337–352. Stone J. (1978) The number and distribution of ganglion cells in the cat’s retina. J. Comp. Neurol. 180, 753–772. Stone J., Rapaport D. H., Williams R. W. and Chalupa L. M. (1982) Uniformity of cell distribution in the ganglion cell layer of prenatal cat retina: implications for mechanisms of retinal development. Dev. Brain Res. 2, 231–242. Williams R. W. (1983) Consequences of prenatal unilateral enucleation upon the visual field topography of the dorsal lateral geniculate nucleus. In Prenatal Development of the Visual System, Dissertation, University of California. Williams R. W. and Chalupa L. M. (1982) Prenatal development of the retinocollicular projection in the cat: an anterograde tracer transport study. J. Neurosci. 2, 604–622. Williams R. W. and Chalupa L. M. (1982) The effects of prenatal unilateral enucleation upon the functional organization of the cat’s lateral geniculate nucleus. Physiologist 25, 223. Williams R. W. and Chalupa L. M. (1983a) Development of the retinal pathway to the pretectum of the cat. Neuroscience 10, 1249–1267. Williams R. W. and Chalupa L. M. (1983b) Expanded retinogeniculate projections in the cat following prenatal unilateral enucleation: functional and anatomical analyses of an anomolous input. Neurosci. Abstr. 9, 701. Williams R. W., Bastiani M. J. and Chalupa L. M. (1983) Loss of axons in the cat optic nerve following fetal unilateral enucleation: an electron microscopic analysis. J. Neurosci. 3, 133–144. Williams R. W., Bastiani M. J. and Chalupa L. M. (1983) Addition and attrition of axons in the optic nerve of the fetal cat: Appearance of growth cones and necrotic fibers. Invest. Ophthal. Vis. Sci., Suppl. 23, 8. Accepted 27 January 1984
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neurogenetics at University of Tennessee Health Science Center
| Print Friendly | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org