|
| |||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Note to the Reader Download the Journal of Neuroscience PDF version of this paper
Print Friendly Common genetic polymorphisms—as opposed to rare mutations—generate
almost all heritable differences in the size and structure of the CNS.
Surprisingly, these normal variants have not previously been mapped or
cloned in any vertebrate species. In a recent paper (Williams et al.,
1996a) we suggested that much of the variation in retinal ganglion cell
number in mice, and the striking bimodality of strain averages, is caused
by one or two quantitative trait loci (QTLs). To test this idea, and to
map genes linked to this variable and highly heritable quantitative trait,
we have counted ganglion cells in 38 recombinant inbred strains (BXD and
BXH) derived from parental strains that have high and low cell numbers. A
genome-wide search using simple and composite interval mapping techniques
revealed a major QTL on chromosome (Chr) 11 in a 3 cM interval between
Hoxb and Krt1 (LOD = 6.8, genome-wide P < 0.001), and possible subsidiary
QTLs on chromosomes 2 and 8. The Chr 11 locus, neuron number control 1
(Nnc1), accounts for one-third of the genetic variance among BXH strains
and more than half of that among BXD strains, but Nnc1 has no known
effects on brain weight, eye weight, or total retinal cell number. Three
strong candidate genes have been mapped previously to the same region as
Nnc1. These genes—Rara, Thra, and Erbb2—encode receptors for retinoic
acid, thyroxine, and neuregulin, respectively. Each receptor is expressed
in the retina during development, and their ligands affect the
proliferation or survival of retinal cells. The most conspicuous differences between the brains of different
mammalian species are quantitative (2,
3). Total brain weight, the size of different brain nuclei, and
numbers of neurons can vary over two or three orders of magnitude (4).
This marked variation ultimately traces back to differences that are
generated, selected, and propagated within single species. Two impressive
examples of variation in the human CNS include the three-fold difference
in the density of cone photoreceptors in the fovea (5)
and the three-fold differences in the total area of both primary and
secondary visual cortex (6).
Some of this variation is undoubtedly environmental, but much is generated
by the independent segregation of alleles that control proliferation,
migration, differentiation, and survival of neurons and glial cells. None
of the genes that are responsible for this normal variation in the
mammalian CNS structure have yet been mapped or identified. Yet these
genes are particularly important because they ultimately influence the
performance and behavioral repertoire of a species. Genetic variation in complex traits is thought to be generated by large
numbers of loci that generally have comparatively small effects (7).
However, a subset of these loci have surprisingly large individual
effects. For example, single quantitative trait loci (QTLs) have been
shown to account for 20-40%; of the variance in the height of corn and the
weight of tomatoes (8).
Similarly, several QTLs individually account for as much as 10%-20%; of
the total variance in numbers of sensory bristles in fruit flies. Some of
these QTLs are now known to correspond to key neurogenic or proneural
genes, including achaete-scute, atonal, enhancer of split, hairy,
Notch, and scabrous (9).
To map genes that contribute to normal variation in the vertebrate CNS,
we have focused on an important and well-defined class of sensory neurons
called retinal ganglion cells. Axons of these neurons give rise to the
optic nerve and are essential for transmitting visual information to the
thalamus and midbrain. Variation in ganglion cell number is generated
primarily by genetic factors (heritability is approximately 0.8) (1).
The distribution of ganglion cell number is close to normal, with a mean
of 60,000 and a range from 40,000 to 80,000. In this respect, variation in
ganglion cell number is a typical complex trait displaying continuous
variation over a wide range. However, one surprising finding from our
previous work is that the distribution of inbred strain averages—as
opposed to individual values—is distinctly bimodal, with modes near 55,500
and 63,500 (1).
This pattern could be generated by the segregation of high and low alleles
at a major QTL. In this study we have mapped this QTL to a gene-rich
region on Chr 11 between Hoxb and Krt1. This is the first
locus known to control normal variation in CNS structure in a vertebrate.
Fixation and processing of tissue. Eyes, optic nerves, and
brains were taken from 182 cases belonging to the 26 BXD strains, 66 cases
belonging to the 12 BXH strains, and 45 cases from the three parental
strains. All mice were obtained from the Jackson Laboratory, Bar Harbor,
Maine. Mice of both sexes and a wide range of age (30 to 400 days) were
anesthetized with an injection of avertin (0.5–0.8 ml ip) and were
perfused transcardially with phosphate buffered saline followed by 1.25%;
glutaraldehyde and 1.0%; paraformaldehyde in 0.1 M phosphate buffer, and
then by 2.5%; glutaraldehyde and 2.0%; paraformaldehyde in 0.1 M PB.
Nerves were dissected, osmicated, and embedded in Spurr's resin. Brains,
including olfactory bulb, were dissected, and weighed. Thin sections of
one or both nerves were placed on formvar-coated slot grids and were
stained with uranyl acetate and lead citrate. The nerves were examined and
photographed on an electron microscope using a systematic sampling
protocol1. C3H/HeJ and 8 of the BXH strains (2, 3, 4, 7, 8, 9, 14, and 16) are all
homozygous for the photoreceptor degeneration allele, rd1, at the beta
phosphodiesterase locus on Chr 5. Despite a massive loss of photoreceptors
during the first two months of life, this mutation has no detectable
effect on the retinal ganglion cell population (r = –0.3). The eight
strains that have the rd1 allele have 61,500 +/- 2000 (SE) ganglion cells,
whereas the four strains that have the wildtype allele have 58,300 +/-
4400 (SE) ganglion cells. Phenotyping. Each ganglion cell has a single axon that extends
from the retina into the brain1, 53. It is therefore possible to count
this cell population simply by counting nerve fibers in single
cross-sections of the optic nerve. A counting frame was traced on
negatives with a marker and all axons within the frame and intersecting
the upper and right edges were marked and counted on the negative using
stereological counting rules. To ensure that unmyelinated fibers were
recognized, negatives were counted while wearing magnifying glasses. The
effective magnification was above X25,000. Approximately 90 cases were
independently replicated. All data were entered into a spreadsheet (Excel
5.0, Microsoft), available at http://www.nervenet.org/main/maincontrol.html.
The average density of axons was multiplied by the area of the nerve
cross-section to estimate the total axon population. Strain averages are
presented as unweighted means. To perform linear regression and compute
residuals we used the program DataDesk 5.0 (Data Description). All
individual brain weights were initially adjusted to that expected of
females weighing 22 gm (54).
This improves the quality of comparisons across strains. A regression
analysis of the stain averages of brain weight and neuron number was then
carried out to compute the residuals listed in
Tables 1 and
2. Analysis of retinas. A 1-mm-wide strip of retina and pigment
epithelium, extending from the head of the optic nerve to the inferior ora
was cut out of one eye from each of 16 cases and embedded flat in Spurr's
resin. The 1-µm-thick sections were cut along the radial axis, mounted,
and stained with hematoxylin. Slides were coded, but were otherwise left
unlabeled. Complete central-to-peripheral cross-sections of the ventral
retina were drawn at low power. The radial depth of cells in the inner and
outer nuclear layers was subsequently determined at 7 to 11 evenly spaced
sites along all sections at X400 using differential interference contrast
optics. Ambiguity of these counts at single sites is less that ± 2 cells.
The outer nuclear layer is between 5 and 15 cells deep, whereas the inner
layer is between 2 and 6 cells deep. The average coefficient of variation
within a case for these measurements was 5.8%; in the outer layer and
7.5%; in the inner layer. QTL mapping. Simple and composite interval mapping (55-57)
was performed using the program Map Manager QT58 and a dataset of RI
strain genotypes compiled by R. W. Elliott and B. Taylor. The original BXD
file is comprehensive and contains many groups of linked loci that have
identical strain distribution patterns. All uninformative and incompletely
typed loci, and loci with numerous unexplained double recombinants over
short intervals were deleted from the dataset. The final BXD database used
for interval mapping and permutation analysis contained 580 completely
typed loci that defines a genome with a total length of approximately
1,640 cM. Genome-wide signficance was estimated by comparing the peak
likelihood ratio statistic (LRS = 4.6 x Lod) of the correctly ordered data
with those computed for 1,000 to 10,000 random permutations of the data59.
To combine probabilities from the two RI sets we computed the probability
associated with a chi-square value equal to -2* (lnPBXD+lnPBXH) with 4
degrees of freedom, where lnPBXD and lnPBXH are the natural logarithms of
the probabilities derived independently for the two RI sets. We have
mapped both the average number of retinal ganglion cells and the residual
number of ganglion cells after eliminating effects of variation in sex,
body weight, brain weight, and age. Files used for mapping are available
at http://www.nervenet.org/main/maincontrol.html. Mapping data have been
deposited with the Mouse Genome Database. Two groups of recombinant inbred strains were studied—the set of 26 BXD
strains generated by crossing C57BL/6J to DBA/2J mice, and the set of 12
BXH strains generated by crossing C57BL/6J to C3H/HeJ mice. The maternal
strain, C57BL/6J, is common to both BXD and BXH RI sets. C57BL/6J (or B
for its black coat color) belongs to the low-cell-number group and has a
population of 55,400 ± 800 ganglion cells (n = 26). Both paternal strains,
DBA/2J (D) in the case of the BXD RI set and C3H/HeJ (H) in the case of
the BXH set, belong to the high group and have populations of 63,400 ±
1,200 (n = 13) and 67,000 ± 1,700 (n = 6), respectively.
The average ganglion cell population in the BXD strains extends from a
low of 50,800 ± 1,100 in BXD27 to a high of 75,800 ± 2,000 in BXD32 (Table
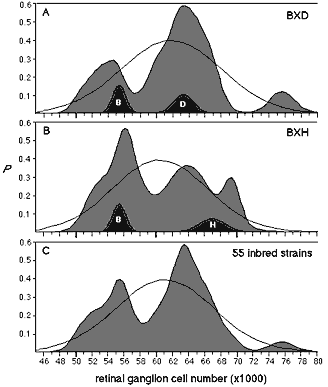
1). The probability density function for the 26 BXD strains has modes
at 54,000 and 64,000 (1a).
These modes correspond almost precisely to the means of the parental
strains and are aligned with the prominent modes discovered in our
previous analysis of 17 common inbred strains (see
Fig. 4 of
Ref. 1). Four strains—BXD1, BXD11, BXD20, and BXD21—have averages that
are in the central range (59,900 to 61,000), reasonably close to the
midparental value of 59,000. Strains BXD5 and BXD32, both of which have
very high cell number, represent a third mode. Average cell number in the
set of BXH RI strains extends from 51,000 to 70,000, without significant
transgression above or below the parental values (Table
2). The probability density function for these strains is broad and is
characterized by a prominent peak at approximately 56,000 and poorly
resolved modes at 64,000 and 70,000 (Fig.
1b). In fully homozygous RI lines the independent assortment of n diallelic
loci gives rise to a maximum of 2n genotypes (e.g., if n = 2
then the genotypes are aabb, aaBB, AAbb, AABB), and each genotype
is expected to be represented equally in the set of RI strains. If a large
number of loci with intermediate or small additive effects assort
independently during the generation of BXD and BXH strains, the
probability densities in figure 1 would tend to have a unimodal and
perhaps Gaussian form. In contrast, the broad and multimodal distributions
of the RI sets, and the clearly bimodal distribution of the data based on
all 55 inbred strains (Fig.
1c), suggest that a small number of QTLs have major effects on neuron
number (note
1). This is consistent with our finds in F2 intercrosses, in which the
number of effective factors controlling ganglion cell number has been
found to be less than three (10).
A comparision of variance within and among these isogenic strains
indicates that genetic factors account for approximately 70%; ± 10%; of
the total phenotypic variance in the RI strains (1,
11). The distribution of phenotypes listed in
Tables 1 and
2 can be compared directly to
those loci that have already been mapped by hundreds of investigators over
the past decade. The single best match on the densely mapped set of BXD
strains is to the tissue specific transplantation antigen 91A gene (Tstap91A)
located approximately 2 cM distal to Hoxb on Chr 11 (12).
Seven of the strains that have low cell number correspond to the C57BL/6J
alleles (B for short) at Tstap91A, and 15 that have high cell
number correspond to DBA/2J alleles (D for short). The four remaining
strains with intermediate phenotypes correspond to 2 B and 2 D genotypes
at Tstap91A. Only one strain, BXD31, is unequivocally discordant.
The correlation coefficient between neuron number and alleles at
Tstap91A is 0.69. (To compute this correlation, B alleles at Mendelian
loci are arbitrarily assigned a value of 0, and D alleles are assigned a
value of 1.) The Lod score for linkage of retinal ganglion cell number
with Tstap91A is 3.7 (Table
3). The probability of achieving this statistic by chance is 0.000037
for a test against a single marker and 0.06 for multiple tests covering
the entire genome. These values underestimate the actual strength of linkage because
variation in brain weight obscures variation in neuron number associated
with the Chr 11 QTL. This global influence can be minimized by computing
residuals after regressing cell number against brain weight (Table
1, right-most column). Mapping these residuals results in a
significant improvement in the strength of linkage (Lod 4.4, single-point
P = 0.0000065, genome-wide P <0.05). Two other chromosomal intervals—one
on Chr 2, the other on Chr 8—were shown to be well correlated with the
remaining genetic variation in ganglion cell number (see below). We
subsequently corrected for the effects of these two intervals and for that
of a third interval on proximal Chr 11 near Glns-ps1 that has been
previously shown to have a significant effect on brain weight among BXD
strains13. In the final analysis the Lod for linkage between cell number
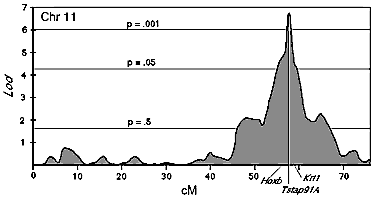
and the Tstap91A interval is approximately 6.8 (Fig.
2, P = 2.0 x 10 E -8, genome-wide P < 0.001). We have named this major
effect QTL on Chr 11 Neuron number control 1 (Nnc1). Nnc1
maps between the Hoxb complex and Mpmv8, an interval of
approximately 3 cM (Fig.
2). The probability of linkage drops more than 100-fold outside of
this interval. Independent support for linkage is provided by the BXH
strain data, in which one of the strongest associations between H alleles
and strains with high cell population (r = +0.58) is also on mid-distal
Chr 11 between Scya and Krt1 (P = 0.01). An analysis of the 12 BXH strains shows that much of the variation in
neuron number in this RI set could be accounted for by a QTL on Chr 4. The
correlation between alleles at Ssdh1 on Chr 4 and cell number is
tight but negative (r = -0.92; Lod is 4.8; P = 2.8 x 10 E-6). Similar
statistics are obtained when mapping residuals that control for the
negative correlation between brain weight and neuron number. Despite these
persuasive statistics, we have good reasons to believe that this linkage
is spurious. First, B alleles inherited from the parental strain with low
cell number are consistently associated with RI strains with high cell
number. Such a reversal, while not uncommon in mapping QTLs that have
modest effects, is unexpected for a QTL with such a massive apparent
effect (r2 = 0.8). Perhaps more telling is the observation that the strain
distribution pattern at Ssdh1 on Chr 4 is almost precisely the same
as that of Rasl5-2 on Chr 4 (11 of 12 common alleles), and nearly
opposite to that of Scya3 (10 of 12 reversed alleles)—a locus
within 10 cM of Tstap91A on Chr 11. Given the small number of BXH
strains and the high density of typed loci, false associations are common.
The set of 12 BXH strains is too small to map loci with any confidence,
but data from small RI sets can be combined to add power and resolution to
a QTL analysis.
After controlling for variance associated with Nnc1 on Chr 11,
two additional intervals that may contain QTLs that modulate cell number
were highlighted in both RI sets. The first is located near Lpl and
Cpe on Chr 8 (approximately 33 cM). The combined Lod for both RI
sets in this region is 2.8 (single-point P = 0.0004). The second interval
is between B2m and Mltr10 on Chr 2 and has a combined Lod of
2.4. (single-point P = 0.001 at Hdc). Clearly, the statistics are
not strong enough to claim QTLs in either interval. As mentioned above,
both regions were subsequently taken into account in composite interval
mapping of the Chr 11 locus. This resulted in a significant improvement in
linkage to the Tstap91A interval on Chr 11. Collectively as much as
70%; of the variation in neuron number and as much as half of the total
phenotypic variance can be accounted for by Nnc1 and by putative
secondary QTLs on Chrs 2 and 8. The phenotypic effects of alleles at single loci are difficult to
estimate from sets of RI strains, and estimates tend to be much too high (14).
However, in this case, the clear separation between high and low strains,
shown particularly well in
Fig. 1c, provides a way to estimate effects of allele substitutions.
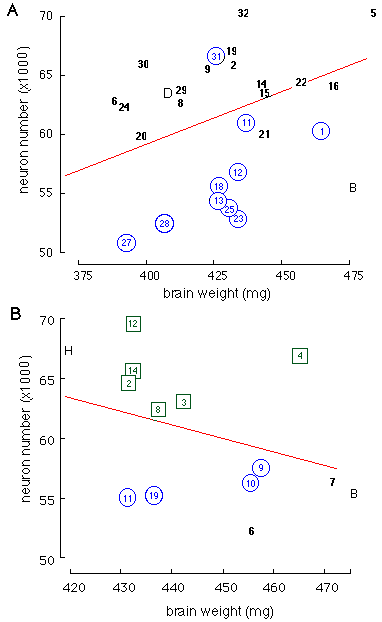
Among BXD strains the substitution of both B alleles with D alleles at
Nnc1 is associated with an increase of approximately 10,000 ganglion
cells. The intermediate population size of F1 progeny from strains with
high and low cell numbers indicates that the mode of gene action is
largely additive (1).
Variation in neuron number is often correlated positively with
variation in brain weight (15,
(16).
The correlation across the BXD and parental strains is +0.54, a highly
significant value (r2 = 0.29, F [1,24] = 10.0, P = 0.004). The polarity of
phenotypes of the parental strains is reversed compared to that for
ganglion cell number: C57BL/6J has lower mean cell number but has a
substantially heavier brain than DBA/2J: 476 ±; 3 mg versus 392 ±; 5 mg.
However, alleles at marker loci close to Nnc1 do not correlate well
with brain weight (r2 = 0.13 at Tstap91A). Furthermore, in the BXH
set, the correlation between brain weight and neuron number is negative (r
= -0.3). This indicates that QTLs controlling variation in retinal
ganglion cell number do not have notable effects on brain weight and
therefore do not have global effects on neuron number in the CNS. However,
given the large number of distinct cell populations in the CNS, Nnc1
may well have pleiotropic effects on other CNS populations.
We have begun to assess the the specificity of Nnc1 within the
eye and retina (17).
Variation in the size of the ganglion cell population does correlate
positively with eye weight in BXD strains (r = 0.55) and retinal area (r =
0.52). However, as is true for brain weight, there is no signficant
correlation between eye weight and alleles at loci on mid-distal Chr 11.
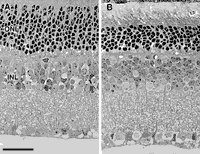
To determine whether Nnc1 affects other cell populations in retina,
we counted cells within the inner and outer plexiform layers of C57BL/6J,
DBA/2J, BXD9, BXD13, BXD22, BXD23, BXD28, and BXD32 mice. There are large
differences between cases and strains—from a low of 6.2 ± 0.3 cells per
radial column in the photoreceptor layer in a BXD32 case with a ganglion
cell population of 85,600, to 11.5 ± 0.5 cells in a BXD28 case with a
ganglion cell population of 43,600. The correlation coefficient between
ganglion cell number and the cell depth of the photoreceptor layer is
-0.32 (95%; confidence interval of r is -0.71 to +0.21). However, the
correlation coefficient between numbers of ganglion cells and cells in the
inner nuclear layer (amacrine, bipolar, horizontal cells, and Muller glial
cells) is +0.53 (confidence interval from +0.05 to +0.81). Collectively,
these correlations suggest that Nnc1 may have effects on cell
number in the inner nuclear layer but does not have effects on
photoreceptor number. We have mapped a major QTL that has a remarkably large effect on the
size of the retinal ganglion cell population in mice. Replacing both
C57BL/6J alleles with DBA/2J alleles at the Nnc1 locus generates a
difference of about 10,000 cells—a 20%; increase in neuron number. The
large effect has allowed us to map this QTL using a modest number of
recombinant inbred strains. One of the principal advantages of recombinant
inbred strains for this type of quantitative analysis is the ability to
reduce non-genetic sources of variance by repeatedly phenotyping the same
genotype. A second key advantage is that the genetic variance among a set
of RI strains is 2- to 4-fold greater than that among corresponding
intercross or backcross progeny (note
2). This improves the power of a search for QTLs. Several investigators (18-20)
have suggested that RI strains be used primarily to highlight chromosomal
intervals that might be worth subsequent analysis using larger panels of
backcross and intercross progeny. We suspect, however, that QTLs with
sufficiently large effects may prove to be more common than expected when
narrowly defined morphological traits are targeted for analysis (18,
19,
21). An alternative strategy that may prove productive is to increase
the number of RI lines included in an analysis and to pool across
partially or completely independent RI sets. In our case, the first 12 BXD
strains that we studied highlighted several candidate intervals, including
mid-distal Chr 11. The addition of the remaining 14 BXD strains winnowed
the initial list of candidates and greatly strengthened linkage near
Tstap91A. Adding the 12 BXH recombinant strains enabled us to detect
secondary QTLs that we would otherwise have missed. By controlling for the
effects of QTLs detected initially in one or the other set of RI strains,
we were able to greatly improve the strength of linkage to the Nnc1
locus. This bootstrap procedure may be particularly useful for mapping
quantitative traits already known to differ substantially among the 10 to
20 strains from which recombinant inbreds have been generated. Nnc1 maps between Hoxb and Krt1 (12).
This region (22)
contains three excellent candidates for Nnc1—Rara, Thra, and
Erbb2. All three genes encode receptors known to be expressed in
retina early in development. It is also known that changing the
concentrations of the ligands of these receptors—retinoic acid, thyroxine,
and neuregulin—affects the proliferation and survival of retinal cells (23-30).
For example, an increase in thyroxine triggers the production of new
retinal ganglion cells that specifically have uncrossed projections in
Xenopus (31).
Retinoic acid has also been found to have effects on retinal cells. For
example, the addition of exogenous retinoic acid increases rod production
at the expense of amacrine cells (29).
Finally, neuregulin, a ligand that activates the erbB2 tyrosine kinase
receptor (32),
promotes ganglion cell survival in culture (24).
To assess the developmental mechanisms generating variation in retinal
ganglion cells among strains we have counted these cells at birth, before
the onset of naturally occurring cell death. Our results suggests that
Nnc1 affects ganglion cell production rather than ganglion cell death
(33).
A growing number of loci are already known to influence numbers and
ratios of retinal cell types when mutated, knocked out, or overexpressed.
The list includes pearl (34),
Brn3 (35),
Pax6 (36),
Mitf (37,
38), Chx10 (39)
Hes1 (40),
Bst (41),
Notch1 (42),
Ccnd1 (43),
Bdnf (44),
Fgf (45),
Ngf (46),
and Bcl2 (47-49).
The loss of Brn3b, for example, reduces ganglion cell numbers by 60
to 70%. Brn-3b has been shown to ge down-regulated by retinoic acid
(58).
In contrast, overexpression of Bcl2 attenuates normal cell death,
allowing twice the normal number of ganglion cells to survive. It is
possible that normal alleles at these loci have more subtle effects and
could account for some of the normal genetic variance not produced by
alleles at Nnc1. Surprisingly, loss of the alpha-1 isoform of the
retinoic acid alpha receptor, one of the candidates for Nnc1, has
no known effect on the eye or retina (50).
Mutant and null alleles at this and other retinoic acid receptors may have
more subtle quantitative effects, a possibility that we are now testing.
The fact that so many null mutants are viable and apparently normal has
led to the idea that key developmental mechanisms are often controlled by
the products of several closely related genes. Some of these redundant
loci may function primarily as QTLs and maintain a reservoir of allelic
variants. The remarkable speed of brain evolution in response to shifts in
selective pressure (4,
15,
51) is dependent upon allelic variants at loci that control the size
of neuron populations by proliferation and cell death (4,
15). The four-fold increase in the size of the cerebellar cortex (52)
that has occurred over the past several million years in the lineage
leading to modern humans was probably brought about by gene modifications
that have increased proliferation in select groups of rhombencephalic
progenitor cells. The rapid reduction in neuron number in the cat's retina
and dorsal lateral geniculate nucleus over a period of less than 20,000
years was probably brought about by changes in severity of natural cell
death (15).
This study has demonstrated that a focused approach that exploits normal
variation can uncover polymorphic loci with large and specific effects on
CNS cell populations. We anticipate that rapid progress in mapping QTLs
with prominent effects on CNS traits will lead to a better understanding
of the basis of normal variation in CNS structure and function, and
ultimately, will lead to a better understanding of the genetic basis of
brain evolution. Acknowledgments. We thank K. Troughton, R. Cushing, and T. Hurt
for technical support. We thank Dr. K. Manly for his program, Map Manager
QT, and Drs. B. Taylor, J. Cheverud, and R. Elliott, and D. Rice for
comments on drafts. This work was supported in part by grants from the
National Institute of Neurological Disease and Strokes (to R.W), and the
National Eye Institute (to R.W. and D.G.). REFERENCES 1. Williams, R.W., Strom,
R.C., Rice, D.S. & Goldowitz, D. Genetic and environmental control of
variation in retinal ganglion cell number in mice. J. Neurosci. 16,
7193-7205 (1996). 2. Haug, H. Brain sizes,
surfaces, and neuronal sizes of the cortex cerebri: a stereological
investigation of man and his variability and a comparision with some
mammals (primates, whales, marsupials, insectivores, and one elephant).
Am. J. Anat. 180, 126-142 (1987). 3. Williams, R.W. & Herrup,
K. The control of neuron number. Annu. Rev. Neurosci. 11, 423-453
(1988). 4. Finlay, B.L. &
Darlington, R.B. Linked regularities in the development and evolution of
mammalian brains. Science 268, 1578-1584 (1995). 5. Curcio, C.A., Sloan Jr.,
K.A., Packer, O., Hendrickson, A.E. & Kalina, R.E. Distribution of cones
in human and monkey retina: individual variability and radial asymmetry.
Science 236, 576-582 (1987). 6. Gilissen, E. & Zilles,
K. The calcarine sulcus as an estimate of the total volume of the human
striate cortex: a morphometric study of reliability and intersubject
variability. J. Brain Res. 37, 57-66 (1996). 7. Lai, C., Lyman, R.F.,
Long, A.D., Langley, C.H. & Mackay, T.F.C. Naturally occurring variation
in bristle number and DNA polymorphisms at the Scabrous locus of
Drosophila melanogaster. Science 266, 1697-1702 (1994). 8. Tanksley, S.D. Mapping
polygenes. Annu. Rev. Genet. 27, 205-233 (1993). 9. Mackay, T.F.C. The
genetic basis of quantitative variation: numbers of sensory bristles in
Drosophila melanogaster as a model system. Trends Genet. 11,
464-470 (1995). 10. Strom, R.C., Goldowitz,
D. & Williams, R.W. Mapping quantitative trait loci that control retinal
ganglion cell number using F2 intercross progeny. Soc. Neurosci. Abst.
22, 518 (1996). 11. Hegmann, J.P. &
Possidente, B. Estimating genetic correlations from inbred strains.
Behav. Genet. 11, 103-114 (1981). 12. Watkins-Chow, D.,
Roller, M., Newhous, M.M., Camper, S.A. & Buchberg, A.M. Mouse
Chromosome 11. Mamm. Genome 6, S201-220 (1996). 13. Williams, R.W., Strom,
R.C. & Goldowitz, D. Mapping quantitative trait loci that control normal
variation in brain weight in the mouse. Soc. Neurosci. Abst. 22, 519
(1996). 14. Lynch, M. & Walsh, B.
in Fundamentals of Quantitative Genetics
(http://nitro.biosci.arizona.edu/zbook/book.html) , (Sinauer Assoc.,
San Francisco, in the press). 15. Williams, R.W., Cavada,
C. & Reinoso-Suarez, F. Rapid evolution of the visual system: A cellular
assay of the retina and dorsal lateral geniculate nucleus of the Spanish
wildcat and the domestic cat. J. Neurosci. 13, 208-228 (1993). 16. Zamenhof, S. & van
Marthens, E. Neonatal and adult brain parameters in mice selected for
adult brain weight. Dev. Psychobiol. 9, 587-593 (1978). 17. Zhou, G. & Williams,
R.W. Mapping genes that control variation in eye weight, retinal area,
and retinal cell density. Soc. Neurosci. Abst. 23, (in the press). 18. Buck, K.J., Metten,
P., Belknap, J.K. & Crabbe, J.C. Quantitative trait loci involved in
genetic predisposition to acute alcohol withdrawal in mice. J. Neurosci.
17, (in the press). 19. Kanes, S. et al.
Mapping the genes for haloperidol-induced catalepsy. J. Pharmacol. Exp.
Ther. 277, 1016-1025 (1996). 20. Plomin, R., McClearn,
G.E., Gora-Maslak, G. & Neiderhiser, J.M. Use of recombinant inbred
strains to detect quantitative trait loci associated with behavior.
Behavior Genetics 21, 99-116 (1991). 21. Dains, K., Hitzeman,
B. & Hitzeman, R. Genetics, neuroleptic-response and the organization of
cholinergic neurons in the mouse striatum. J. Pharmacol. Exp. Therap.
279, 1430-1438 (1996). 22. Buchberg, A.H. A
comprehensive genetic map of murine chromosome 11 reveals extensive
linkage conservation between mouse and human. Genetics 122, 153-161
(1989). 23. Beach, D.H. &
Jacobson, M. Influence of thyroxine on cell proliferation in the retina
of the clawed frog at different ages. J. Comp. Neurol. 183, 615-624
(1979). 24. Bermingham-McDonogh,
O., McCabe, K.L. & Reh, T.A. Effects of GGF/neuregulins on neuronal
survival and neurite outgrowth correlate with erbB2/neu expression in
developing rat retina. Development 122, 1427-1438 (1996). 25. Hoskins, S.G. &
Grobstein, P. Induction of the ipsilateral retinothalamic projection in
Xenopus laevis by thyroxine. Nature 307, 730-733 (1984). 26. Hyatt, G.A. et al.
Retinoic acid establishes ventral retinal characteristics. Development
121, 195-204 (1996). 27. Hyatt, G.A., Schmitt,
E.A., Marsh-Armstrong, N.R. & Dowling, J.E. Retinoic acid-induced
duplication of the zebrafish retina. Proc. Natl. Acad. Sci. USA 89,
8293-8297 (1992). 28. Kelley, M., Turner,
J.K. & Reh, T.A. Ligands of steroid/thyroid receptors induce cone
photoreceptors in vertebrate retina. Development 121, 3777-3785 (1995).
29. Kelley, M.W., Turner,
J.K. & Reh, T.A. Retinoic acid promotes differentiation of
photoreceptors in vitro. Development 120, 2091-2102 (1994). 30. Stenkamp, D.L.,
Gregory, J.K. & Adler, R. Retinoid effects in purified cultures of chick
embryo retina neurons and photoreceptors. Invest. Ophth. & Vis. Sci. 34,
2425-2436 (1993). 31. Hoskins, S.G. Control
of the development of the ipsilateral retinothalamic projection in
Xenopus laevis by thyroxine: results and speculation. J. Neurobiol. 17,
203-229 (1985). 32. Meyer, D. & Birchmeier,
C. Distinct isoforms of neuregulin are expressed in mesenchymal and
neuronal cells during mouse development. Proc. Natl. Acad. Sci. USA 91,
1064-1068. (1994). 33. Strom, R.C., Williams,
R.W. & Goldowitz, D. Developmental mechanisms responsible for strain
differences in the retinal ganglion cell population. Soc. Neurosci. Abst.
21, 1523 (1995). 34. Williams, M.A., Pinon,
L.G.P., Linden, R. & Pinto, L.H. The Pearl mutation accelerates the
schedule of natural cell death in the early postnatal retina. Exp. Brain
Res. 82, 393-400 (1990). 35. Gan, L. et al. Pou
domain factor Brn-3b is required for the development of a large
set of retinal ganglion cells. Proc. Natl. Acad. Sci. USA 93, 3920-3925
(1996). 36. Grindley, J.,
Davidson, D. & Hill, R. The role of Pax-6 in eye and nasal
development. Development 121, 1433-1442 (1995). 37. Packer, S.O. The eye
and skeletal effects of two mutant alleles at the microophthalmia locus
of Mus musculus. J. Exp. Zool. 1, 21-45 (1967). 38. Steingrimsson, E. et
al. Molecular basis of mouse microphthalmia (mi) mutations helps explain
their developmental and phenotypic consequences. Nature Genet. 8,
256-263 (1994). 39. Burmeister, M. et al.
Ocular retardation mouse caused by Chx10 homeobox null allele:
impaired retinal progenitor proliferation and bipolar cell
differentiation. Nature Genet. 12, 376-384 (1996). 40. Tomita, K. Mammalian
hairy and enhancer of split homolog 1 regulates differentiation of
retinal neurons and is essential for eye morphogenesis. Neuron 16,
723-734 (1996). 41. Rice, D.S. et al.
Mapping the Bst mutation on mouse chromosome 16: a model for
human optic atrophy. Mamm. Genome 6, 546-548 (1995). 42. Austin, C.P., Feldman,
D.E., Ida, J.A. & Cepko, C.L. Vertebrate retinal ganglion cells are
selected from competent progenitors by the action of Notch. Development
121, 3637-3650 (1995). 43. Sicinski, P. &
Weinberg, R. Cyclin D1 provides a link between development and
oncogenesis in the retina and breast. Cell 82, 621-630 (1995). 44. Johnson, J.E., Barde,
Y.A., Schwab, M. & Thoenen, M. Brain-derived neurotrophin factor
supports the survival of cultured rat retinal ganglion cells. J.
Neurosci. 6, 3031-3038 (1986). 45. Cepko, C.L. &
Guillemot, F. Retinal fate and ganglion cell differentiation are
potentiated by acidic FGF in an in vitro assay of early retinal
development. Development 114, 743-754 (1992). 46. Ribacchi, S.A., Ensini,
M., Bonfanti, L., Bravina, A. & Maffei., L. Nerve growth factor reduces
apoptosis of axotomized retinal ganglion cells in the neonatal rat.
Neuroscience 63, 969-973 (1994). 47. Bonfanti, L. et al.
Protection of retinal ganglion cells from natural and axotomy-incuded
cell death in neonatal transgenic mice overexpressing bcl-2. J. Neurosci.
16, 4186-4194 (1996). 48. Burne, J.F., Staple,
J.K. & Raff, M.C. Glial cells are increased proportionally in transgenic
optic nerves with increased numbers of axons. J. Neurosci. 16, 2064-2073
(1996). 49. Martinou, J.-C. et al.
Overexpression of Bcl-2 in transgenic mice protects neurons from
naturally occurring cell death and experimental ischemia. Neuron 13,
1017-1030 (1994). 50. Lufkin, T. et al. High
postnatal lethality and testis degeneration in retinoic acid receptor a
mutant mice. Proc. Natl. Acad. Sci. USA 90, 7225-7229 (1993). 51. Armstrong, E. Relative
brain size and metabolism in mammals. Science 220, 1302-1304 (1983). 52. Llinas, R.R. & Walton,
K.D. in Synaptic Organization of the Brain (ed G.M. Shepherd) 214-245
(Oxford Univ. Press, New York, 1990). 53. Rice, D.S., Williams,
R.W. & Goldowitz, D. Genetic control of retinal projections in inbred
strains of albino Mice. J. Comp. Neurol. 354, 459-469 (1995). 54. Williams, R.W.,
Goldowitz, D. & Strom, R.C. Brain weight in relation to body weight, age
and sex: A multiple regression analysis. Soc. Neurosci. Abst. 23, (in
the press). 55. Haley, C.S. & Knott,
S.A. A simple regression method for mapping quantitative trait loci in
line crosses using flanking markers. Heredity 69, 315-324 (1992). 56. Jansen, R.C. Interval
mapping of multiple quantitative trait loci. Genetics 135, 205-211
(1993). 57. Tinker, N.A. & Mather,
D.E. Methods for QTL analysis with progeny replicated in multiple
environments. J. Quant. Trait Analysis (1996). 58. Manly, K. (1996).
MapManager QT.
(http://mapmgr.roswellpark.org/mapmgr.html). 59. Churchill, G.A. &
Doerge, R.W. Empirical threshold values for quantitative trait mapping.
Genetics 138, 963-971. Note 1. The first two
modes in Figure 1 can be fit neatly by a single locus additive model
with the substitution of a pair of DBA/2J alleles adding +10,000 cells
to the base population of 54,000. The third mode (BXD5 and BXD32) can be
fit by considering one or two additional QTLs (probably a positive
alleles from the C57BL/6J parental strain) and by assuming significant
non-linear epistatic interactions among loci. All 1-locus, 2-locus, and
3-locus models that we explored required effects of +5,000 to +6,000 per
DBA/2J allele to obtain a good fit. Note 2. While the
advantages of RI strains for mapping quantitative traits that are
subject to substantial non-genetic variance have been clear for many
years, RI strains have rarely been used as the principal method to map
QTLs. The main obstacle has been that the number of RI strains is
usually too small to define QTLs with phenotypes that are normally
distributed or controlled by large numbers of factors. Since 11 August 98
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neurogenetics at University of Tennessee Health Science Center
| Print Friendly | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org